2.8.2021
On February 4, Johnson & Johnson requested Emergency Use Authorization for their COVID-19 vaccine. The Chairman and Chief Executive Officer at the company, Alex Gorsky, stated, “As the world’s largest healthcare company, we are bringing to bear our best scientific minds, and rigorous standards of safety, in collaboration with regulators, to accelerate the fight against this pandemic. This pivotal milestone demonstrates our focused efforts toward a COVID-19 vaccine that is built on collaboration and deep commitment to a robust scientific process.”
This announcement comes as new COVID-19 variants cause concern. Initial findings suggest that the vaccine, which is being developed by Johnson & Johnson subsidiary, Janssen Pharmaceutical Companies, is 85% effective at preventing severe disease across all variants. Although this percentage is slightly less than Pfizer and Moderna’s, the Janssen vaccine had no reported cases of hospitalization and death 28 days post-vaccination, and the easy storage requirements and single-dose regimen will make it easier to distribute and administer than the other two vaccines. As the leader in COVID-19 back-to-work solutions, we outline some key elements about the Janssen clinical trial below.
Study Details
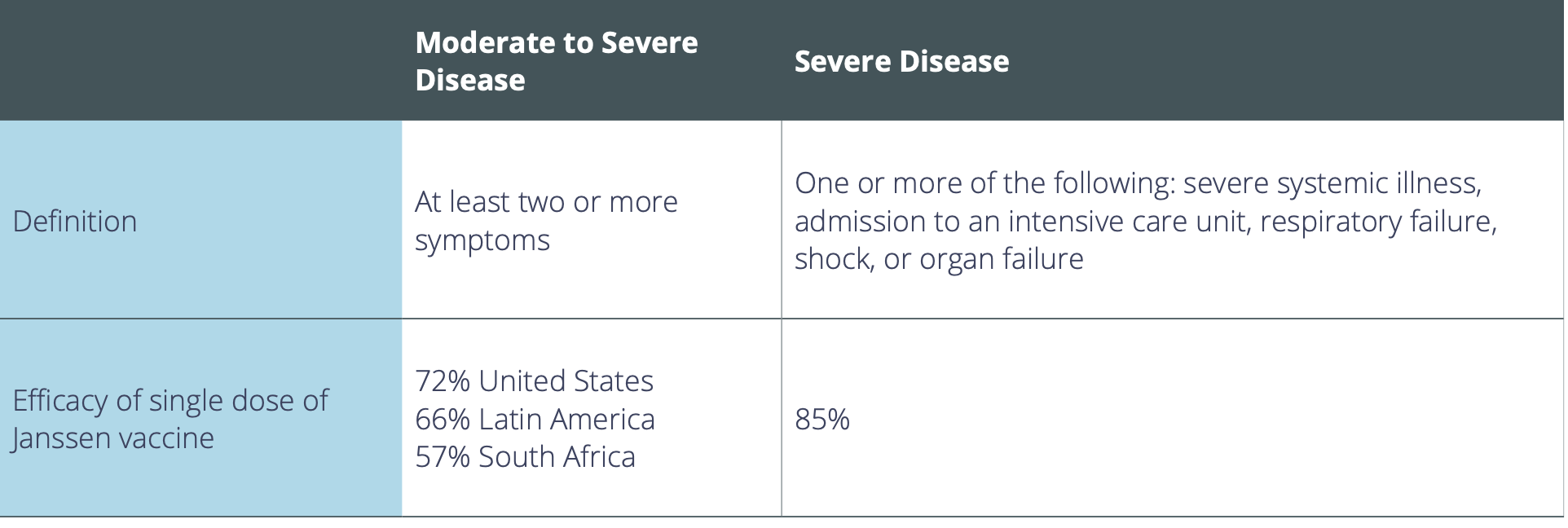
Approximately 43,783 participants enrolled in Janssen’s clinical trial, known as ENSEMBLE. In the study, participants were randomly assigned to either receive the single dose of the vaccine or a placebo injection. Scientists then assessed the individuals at 14 days and 28 days to evaluate their immune responses. Specifically, they analyzed whether the vaccine prevents people from getting sick with moderate to severe COVID-19—defined as exhibiting at least two symptoms, such as a cough and fever—in addition to receiving a positive PCR test.
Similar to Pfizer and Moderna’s studies, the Janssen clinical trial was double-blinded; neither the participants nor the study administrators knew whether volunteers received the vaccine or the placebo. This study design is meant to prevent any biases from impacting the evaluation of the outcomes.
Effectiveness
Overall, the Janssen vaccine was 66% effective in preventing moderate to severe COVID-19 28 days after vaccination. However, the level of protection varied by participant location; the Janssen vaccine was 72% effective in the United States, 66% effective in Latin America, and 57% effective in South Africa. This discrepancy suggests that the vaccine was not as effective in preventing moderate to severe infection in participants who encountered the COVID-19 variant from South Africa.
Nonetheless, the vaccine candidate showed complete protection against COVID-19 hospitalization and death. The vaccine was also 85% effective in preventing the stricter definition of severe disease across all variants and geographies in the study 28 days after vaccination, and the efficacy against severe disease increased over time with no reported cases after day 49. Janssen is also testing whether a second dose would increase the overall efficacy.
Dosage
The Janssen vaccine is currently a single dose. However, they are simultaneously conducting studies to determine if an additional dose will increase its overall effectiveness.
Side Effects
Overall, the side effects reported from the trial were minor and consisted of soreness at the injection site, fatigue, headache, and muscle aches.
The Science Behind the Vaccine
Janssen’s vaccine uses an adenovirus, which is a type of virus that causes the common cold. This viral vector is weakened so that it cannot replicate but instead carries the genetic material via double-stranded DNA to produce the coronavirus’ spike protein. Consequently, the spike protein produces an immune response, enabling your body to fight off any future infections caused by the novel coronavirus. Adenovirus vectors are well studied and have a substantial amount of long-term safety data because they have been utilized for several other vaccines such as HIV, RSV, and Ebola.
Storage
The vaccine can last two years at -4 degrees Fahrenheit, and for at least three months at standard refrigeration temperatures (36 to 46 degrees Fahrenheit) compared to Pfizer and Moderna’s much colder temperatures. The difference in storage requirements is that DNA is not as fragile as RNA, and the adenovirus has a protein coat that protects the genetic material. These requirements make the Janssen vaccine far easier to transport, distribute, and store; and more data is being collected to determine if the vaccine will be able to remain stable for even longer at refrigeration temperatures.
Cost and Availability
Janssen stated that they are “committed to bringing an affordable COVID-19 vaccine on a not-for-profit basis for emergency pandemic use, pending regulatory authorizations.” When the vaccine is available, the US government will provide the vaccine to its citizens for free. If the vaccine is authorized and all goes well with manufacturing and supply chain, Janssen plans to deliver 100 million doses to the US by the end of June.
The FDA has scheduled a public meeting of its Vaccines and Related Biological Products Advisory Committee for February 26. As with Pfizer and Moderna’s vaccines, this independent group of experts will make a recommendation to the FDA based on its review of the data. This vaccine, along with Pfizer and Moderna’s vaccines, would be another tool to help end the pandemic. In the meantime, we encourage everyone to continue to follow CDC guidelines such as social distancing, washing your hands, and wearing a mask.
Disclaimer:
This information is based on current resources available and is subject to change. This document and its contents are provided for informational purposes only, and not intended to be, and should not be understood or treated as, a substitute for professional medical advice around COVID-19, its risks or symptoms, or to take the place of any local, state and national laws and guidelines around COVID-19. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition.